
A hexadecylamide derivative of hyaluronan (HYMOVIS ®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan. Smith MM, Russell AK, Schiavinato A, Little CB.Parsippany, NJ: Fidia Pharma USA Inc 2015. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C.Parsippany, NJ: Fidia Pharma USA Inc 2014. Rheological and molecular weight comparisons of approved hyaluronic acid products - preliminary standards for establishing class III medical device equivalence. Braithwaite GJ, Daley MJ, Toledo-Velasquez D.Pambio-Noranco, Switzerland: Institut Biochimique SA 2016. Parsippany, NJ: Ferring Pharmaceuticals 2016. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. Silver Spring, MD: US Food and Drug Administration 2014. Monovisc summary of safety and effectiveness data.Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. Rheology of joint fluid in total knee arthroplasty patients. Mazzucco D, McKinley G, Scott RD, Spector M.Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Balazs EA, Watson D, Duff IF, Roseman S.View the Complete Prescribing Information for SYNVISC-ONE The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC-ONE: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance. The most commonly reported adverse events were arthralgia, arthritis, arthropathy, injection site pain, and joint effusion. View the Complete Prescribing Information for SYNVISC For SYNVISC-ONE The following reported adverse events are among those that may occur in association with intra-articular injections, including SYNVISC: arthralgia, joint stiffness, joint effusion, joint swelling, joint warmth, injection site pain, arthritis, arthropathy, and gait disturbance. In clinical trials, the most commonly reported adverse events were transient pain, swelling, and/or joint effusion in the injected knee. Patients should be advised to avoid strenuous or prolonged weight-bearing activities for approximately 48 hours after treatment.
The safety and effectiveness of SYNVISC and SYNVISC-ONE have not been established in children (≤21 years old) or in pregnant or lactating women. Strict adherence to aseptic technique must be followed to avoid joint infection.
Remove any synovial fluid or effusion before injecting SYNVISC or SYNVISC-ONE. Use caution when injecting SYNVISC or SYNVISC-ONE in patients allergic to avian proteins, feathers, or egg products who have evidence of lymphatic or venous stasis in the leg to be treated or who have severe inflammation in the knee to be treated. The safety and efficacy of SYNVISC-ONE in locations other than the knee, or for conditions other than osteoarthritis, or in combination with other intra-articular injectables, or in severely inflamed knee joints have not been established.
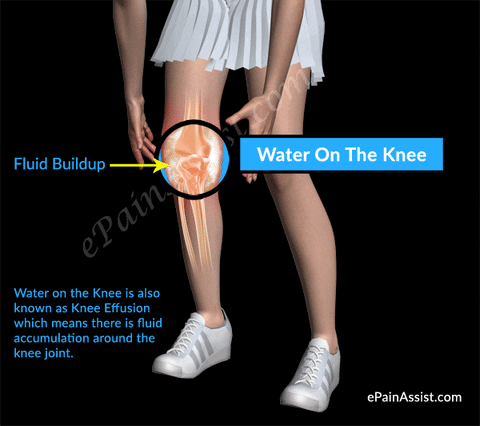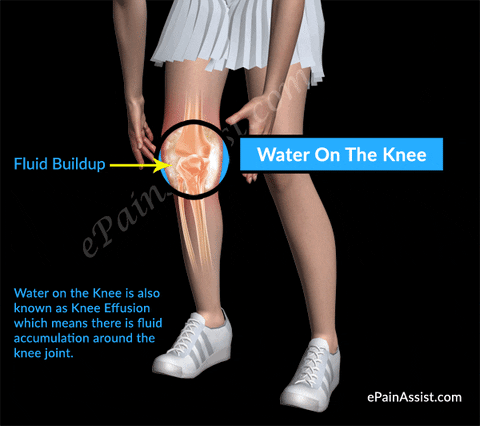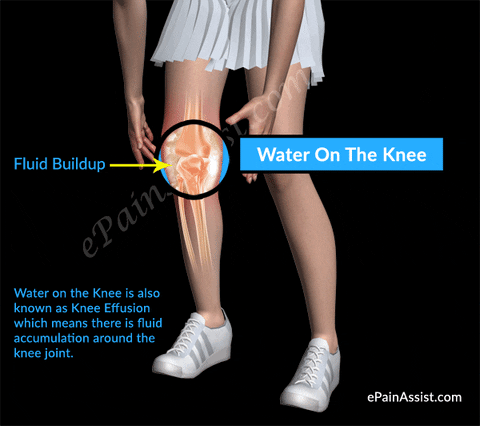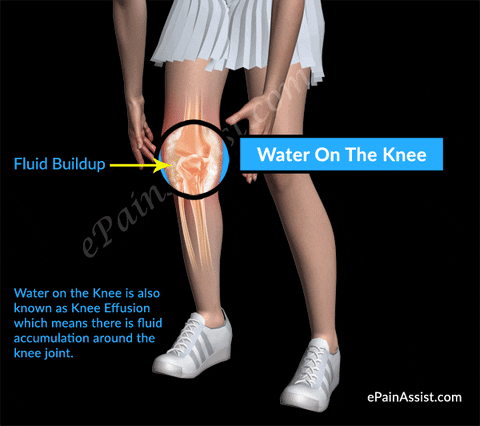
#Injections sunovial fluid in knee skin
Patients should be instructed to contact their treating physician if signs of skin disorder (such as change of color or open sores) appear. Some cases of skin necrosis have been reported after intra-articular use of hyaluronic acid. Do not inject SYNVISC or SYNVISC-ONE extra-articularly, into the synovial tissues, into the fat pad or joint capsule, or intravascularly. Hypersensitivity reactions including anaphylactic reaction, anaphylactoid reaction, anaphylactic shock and angioedema have been reported for both SYNVISC and SYNVISC-ONE.ĭo not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence.

SYNVISC and SYNVISC-ONE are contraindicated in patients with known hypersensitivity to hyaluronan products or patients with infections in or around the target knee.


 0 kommentar(er)
0 kommentar(er)
